Absolutely Unique & Substantially Superior
More Than Subject Recruiters, We Are Patient Advocates
Our team was built from the ground up with the purpose of finding and supporting advanced Parkinson’s disease patients seeking additional treatment options.
What do we do that is above and beyond the competition? |
Them | Us |
|---|---|---|
Movement disorders specialized staff |
||
Review of informed consent by phone with candidate |
||
Detailed screening directly corresponds to the Inclusion/Exclusion Criteria |
||
Phone interview to determine candidate suitability |
||
Principle Investigator Doctor to Doctor capability to discuss candidates |
||
Specifically designed media assets for advanced Parkinson’s disease |
||
Developed database with hundreds of highly likely candidates |
||
Ad campaigns developed specifically for individual trials to engage with the target group (e.g. de novo patients, patients with motor fluctuations, patients with troublesome dyskinesias, etc.) |
||
Telephone warm transfer of most valuable leads to site coordinators |
||
Extensive recruiting experience for PD patients |
||
Fair, balanced and broad phone based advocacy program |
||
Largest PD Facebook Support Group in the world |
||
Over 50,000 monthly pageviews on a PD patient facing website |
||
Medical record acquisition |
Optional | |
Medical record review |
Optional | |
Delivery of patient medical records to sites |
Optional |
Question: Are all doctors the same?
Of course not. Some are specialists some are a generalist. Some doctors are excellent, some are good and unfortunately a few are bad.
The same can be said about digital marketers and subject recruiters. We are excellent digital marketers and patient advocates specialized in clinical trials recruitment for Parkinson’s disease patients. There was a time that Search Engine Optimization and Search Based Marketing was the best digital tool for online communication. That is no longer true for subject recruitment. Search based marketing waits for the patient to research a trial online or begin searching keywords associated with their disease state. Profile-based marketing enabled by social media, allows us to engage patients when they are online doing anything. They could be watching kitty cat videos or messaging a friend when our messages are displayed. Because we are laser-focused on the serving the PD community, we can identify nearly 1 million people in the US that are strongly related to the disease (primarily patients and caregivers.) Our methods of communicating with candidates are absolutely unique and superior.
Search Based
GoogleSearch Based Advertising
This method requires the patient to come to you by searching for a keyword. This is useful but very limiting.
Profile Based
FacebookSocial Media
Targets profiles, not keywords. You go to the patients to deliver the message opposed to waiting for them to ask the question.
47,600,000 Facebook users over the age of 55 in the US as of January 2018
Approximately 92,800,000 people in the US are over the age of 55
Database of interested prospective subjects which can fill many Parkinson’s disease related clinical trials.
Research Catalyst has a substantial database of PD patients that is rapidly growing. The data collection process has been tailored to accommodate anticipated clinical trials. Through the brand Parkinson’s Community Research Catalyst delivers millions of advertising impressions to hundreds of thousands unique people associated with PD in the United States. This generates over fifty thousand web visits each month and a database of nearly twenty thousand people that have answered a clinical trials prescreener with over a hundred specific qualifying questions pertinent to most PD clinical trials I/E criteria.
By The Numbers
Hundreds of candidates that meet even the strictest inclusion and exclusion criteria for most PD trials
Nearly 20,000 PD Profiles Acquired
1 Million Unique Viewers
Millions of Impressions
Access These High-Quality Profiles
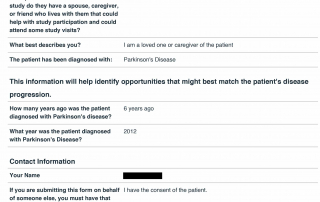
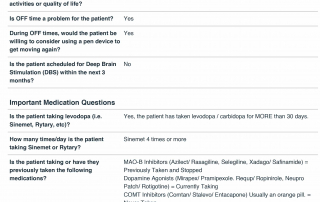
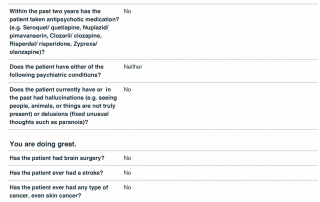
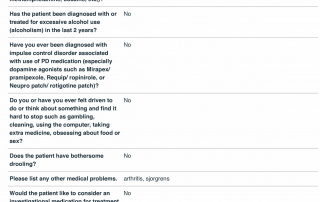
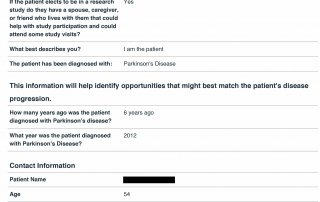
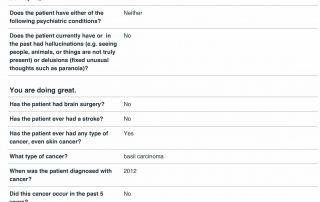
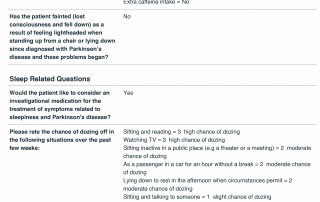
Look at the quality of the candidates in this database as each question is designed to determine the eligibility of candidates based upon the inclusion and exclusion parameters of PD related clinical trials.
Candidate Profile Example 1
Candidate Profile Example 2
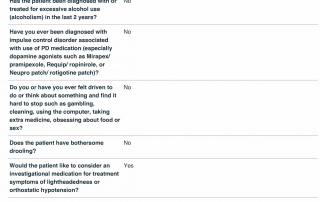
Phone Screening
These questions typically require more advance review by our staff trained in movement disorders clinical trials including research coordinators and a principal investigator.
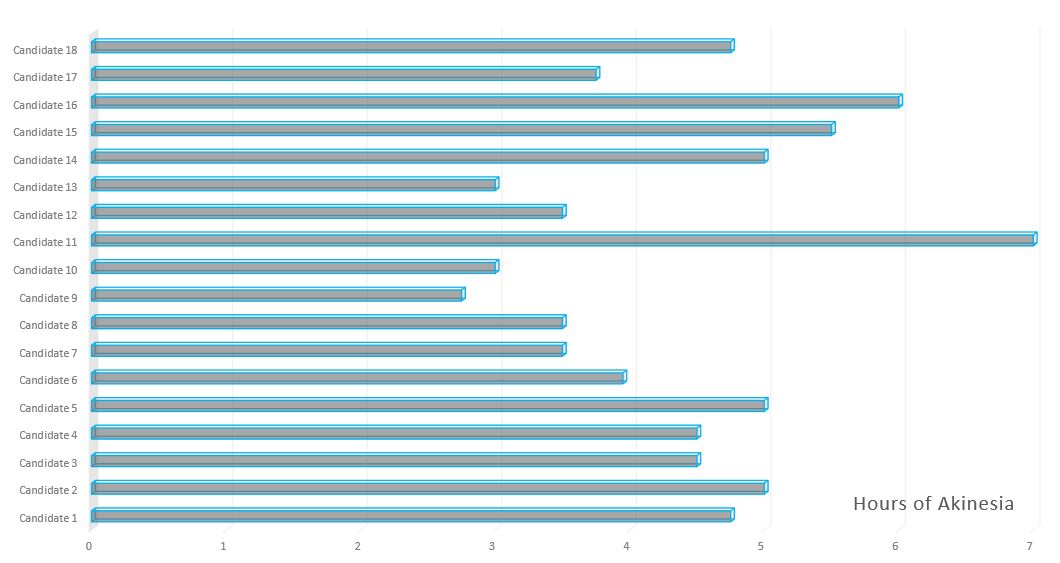
Capability to identify troublesome akinesia.
Research Catalyst’s Patient Advocates conducted a study to determine if the online questions above adequately selects patients that will timeline appropriately at screening for akinesia. 18 consecutive candidates that answered the above questions affirmatively timelined for OFF time severity of akinesia well over 2 hours on their average day. This process takes approximately 45 minutes per candidate as the patient is guided through the day and detailed information is acquired to accurately account for dose time, amount, akinesia and dyskinesia. The results are summarized in this chart.
Determined by Sites Research Coordinator and Principle Investigator
Born from a Research Site
We understand the challenges faced by sites.
We get better site participation because we work differently and do much more. We understand that most sites hate working with central recruitment companies that just waste the time of coordinators and send lots of useless candidates to the site. We only send a few highly qualified candidates to sites. These candidates live close to the site and are highly vetted. Most should be ready to bring in for screening. Our Medical Director (a PI himself), will reach out to the principle investigators at each site to facilitate engagement with the PI’s and their research coordinators and will educate them about our processes about how we work differently than other central recruitment companies.
Performing Well For Clients
Through Doing Right By Patients
Advocacy Process & Service

The Advocacy Program

The advocacy program works. We historically reach 80% of high-quality candidates by phone within 45 days.
Leverage Social Media & Algorithmic Decision Modeling
Digital technology and social media provide new tools to identify and communicate with patients in extraordinary ways. We can now broadcast and interact almost exclusively with very narrow targets, such as people who identify with advanced Parkinson’s disease. We can focus our advertising dollars towards these patients and their loved ones and do not need to expend advertising dollars to deliver messages to a non-applicable audience. Social media opportunities not only offer laser focus but also massive broadcast. Facebook recently celebrated 1 billion active users in a single day. Through the proper use of social media advertising, Research Catalyst can reach nearly 1 million people in the U.S. who identify with Parkinson’s disease as either a patient or a caregiver. HIPAA compliant technology has also advanced to the point that algorithmic decision models can collect patient health information with extreme efficiency. Research Catalyst has developed a process that automatically collects up to 108 pieces of information from a patient as they answer questions to identify their suitability for investigational and approved treatment options. Through technology comes efficiency, and as a result of a more focused and automated screening process, extensive resources can be delivered to the right patients once they have been identified.
Compliance Is Just The Start
We do more than follow the rules; We work with the people who make them and embody their intention. The regulatory environment concerning the use of social media for the promotion of pharmaceuticals is complex. A comprehensive understanding of not only the rules but also the intention of the law is imperative. Research Catalyst approaches the ethical considerations of patient education and marketing from a patient first perspective. All information provided must be accurate, truthful, complete, fair and balanced. The industry standard of fair balance for post FDA marketing generally includes an accurate representation of the indicated use, proven benefits, and potential side effects. We abide by the rules established to govern FDA approved marketing campaigns and adhere to a stricter and more comprehensive set of guidelines designed for the medical research community. The set of principles most relevant to this discussion is, of course, the informed consent process. This ethical framework not only requires accurate and balanced information about a treatment option (indications, potential benefit and risks), but most notably incorporates education about other suitable opportunities the patient should consider that are not associated with the sponsored treatment. Broadly educating the patient about reasonable options such as support groups, exercise, physicians in their area, and other treatment opportunities not only protects our sponsors but also gains the patients’ trust as we prioritize their wellbeing before our financial incentive. We are not only doing what is compliant but also what is right.
This methodology goes beyond what is required by law and actualizes the governing bodies vision as it relates to the incorporation of digital media to the patient education process. By placing the patient first and understanding both the laws and their intentions, Research Catalyst establishes the security to leverage internet technology with unprecedented results. We distance ourselves and our clients in this way from the bleeding edge and stay firmly at the cutting edge. By educating patients broadly, we earn their trust and our message is heard. Integrating the most ethical practices with the most sophisticated technology can produce the greatest reward for both the patient and the organizations that support them.
Resources & Capabilities
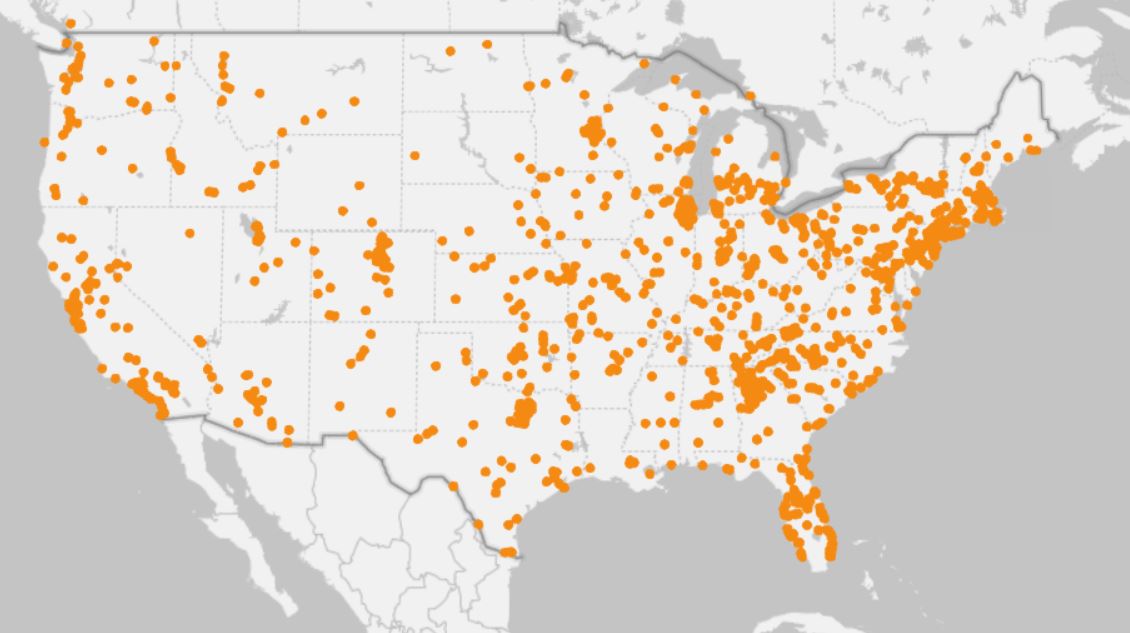
Social Media’s PD Audience Size in the US
Research Catalyst has mapped nearly 1 million people in the United States in to a Parkinson’s disease cohort through Facebook and Instagram and communicates with this population in a big way.


Largest PD Support Group on Facebook with over 20,000 Members
We own, administer and moderate the largest Parkinson’s disease group on Facebook.
This group is an excellent resource that builds trust and allows us to conduct market research quickly.
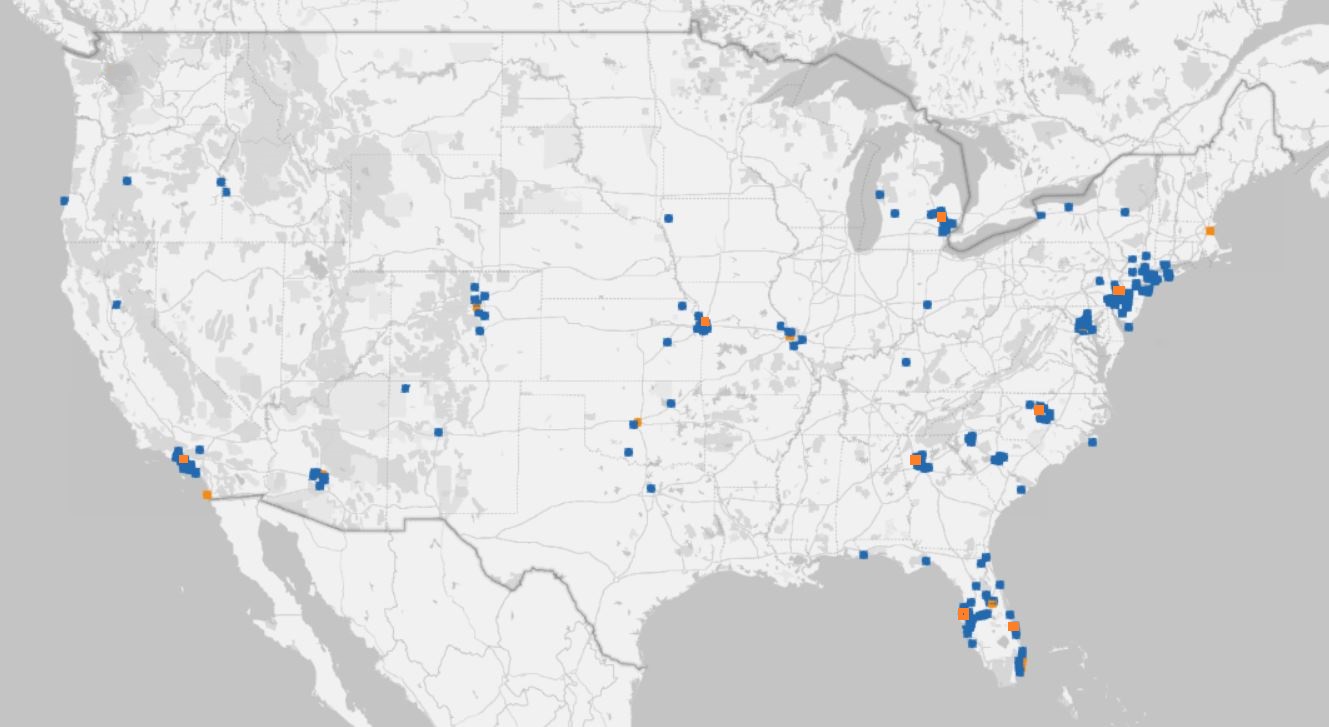
Qualifying Patients By Region to Support Site Initiation Decision Making
We can target communication to people within range of sites and also help prioritize site initiation based on where identified candidates live.
Payment Options
Campaigns can be run concurrently with other recruitment efforts or stand alone. With limited upfront investment required, you can safely increase the chance of completing recruitment on or before schedule with very little exposure.
Estimated Pricing: $5,000 per screen (pricing may vary)
Optional Add On Services: $150 per complete set of medical records, $200 per physician review of a candidate.
- HIPAA compliant digital infrastructure
- Call center services
- Online medical record release form capture
- Automated responsive online pre-screener
Estimated Pricing: $50,000/month
The all-inclusive plan is paid in advance every 4 weeks. This is a stable payment option that mitigates variable cost and always stays within budget. The client is in control to cancel at any time, so we are motivated to not only perform but to keep you updated and informed.
Plan includes all setup and operating costs such as advertising, infrastructure and staffing:
- HIPAA compliant digital infrastructure
- IRB reviewed and approved advertising
- Marketing budget and campaign management
- Call center services
- Online medical record release form capture
- Automated responsive online pre-screener
- Physician review of candidates for placement